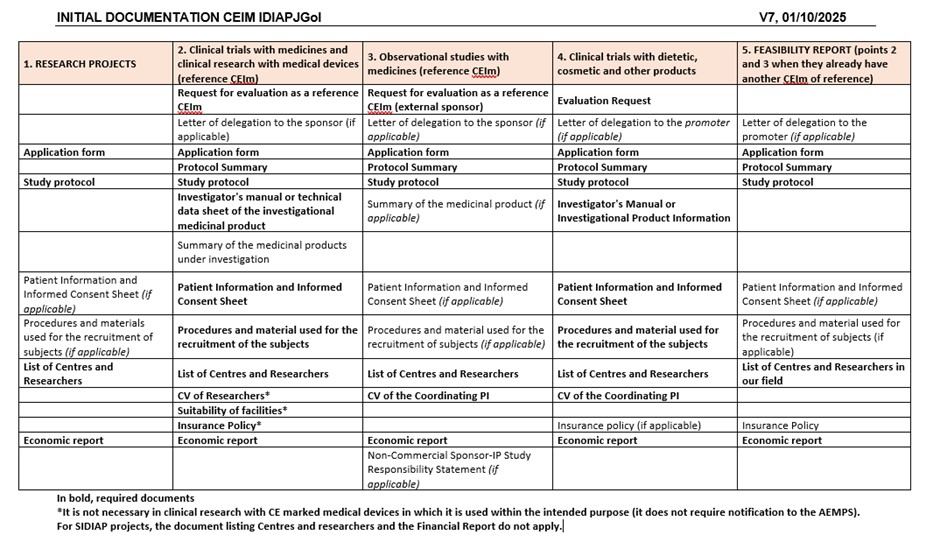
It is necessary to submit a Request Letter to the CEIm IDIAP Jordi Gol when it must act as the reference CEIm for an observational study with medicines (EOm) or a clinical investigation with medical devices.
If the project has no commercial interest, the declaration of responsibility for a non-commercial study must be provided by the sponsor-PI (if applicable).
If a prospective EOm is to be carried out, an evaluation must be requested from the Department of Health before signing the contract, except for studies promoted by a public administration and those in which it is certified that the research is a non-commercial clinical investigation. You can find more information at the following link: https://medicaments.gencat.cat/ca/professionals/recerca/estudis-observacionals-amb-medicaments/